On the Interception of the Optimal Imaging Plane for Pleural Line Scanning with Automatic Robot Assisted Lung Ultrasound: An Experimental Study
Keywords:
Lung ultrasound (LUS), Pleural line (PL), Pleural plane (PP), Robot-Assisted UltrasoundAbstract
Background: Lung ultrasound (LUS) is nowadays an important tool to evaluate the state of lung surface. However, it is strongly operator-dependent, leading to reduced reproducibility of LUS analysis. Even though LUS acquisition protocols can improve LUS reproducibility and help standardizing LUS exams, human operators can guarantee only a limited precision in intercepting the optimal imaging plane. Hence, in this study, we assess the possibility to automatically intercept the optimal imaging plane in LUS examinations, i.e., the imaging plane perpendicular to the pleural plane (PP), by extracting three features, then utilized to guide a UR5e robotic arm handling an ultrasound probe.
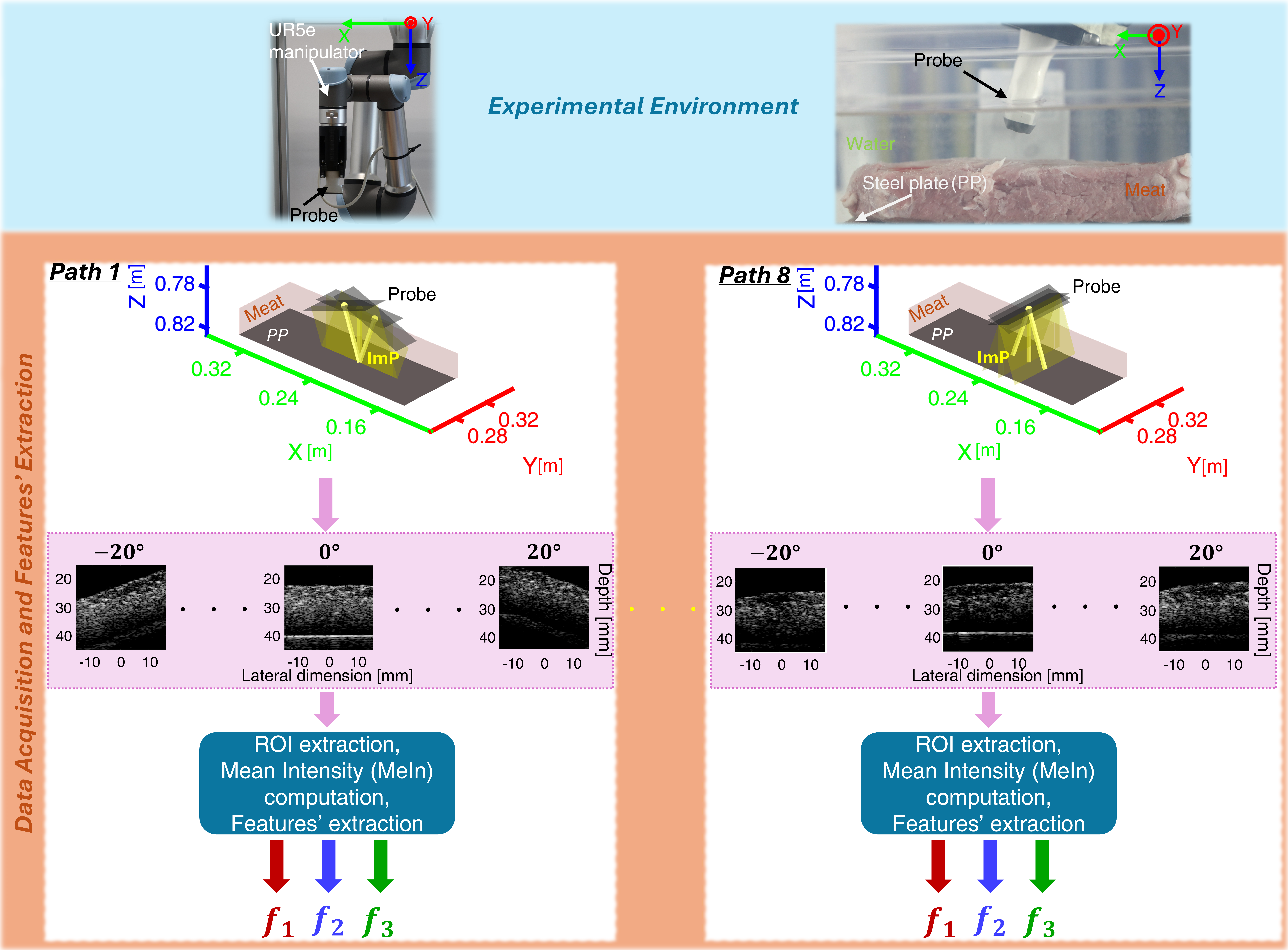
Methods: The main focus of this study consists on evaluating the potential of these three features in estimating the PP position with respect to the probe. To do so, we designed a simplified but highly controllable environment, where PP was mimicked with a steel plate (to simulate a highly reflective acoustic interface), while intercostal tissues were mimicked with a 2-cm-thick beef meat. The environment was imaged with a linear probe connected to an ULA-OP platform, which was held by an UR5e, programmed to explore 8 different paths of acquisitions with a rotational angle (RA) ranging from -20º to 20º (1º step size). This resulted in 328 positions that could be explored; each position with RA=0º corresponds to the optimal imaging plane. Radiofrequency data were acquired and post-processed to form normalized log-scale B-Mode images. A rectangular region of interest, defined to include PP, was considered to compute mean intensity at each depth of the region of interest, along lateral dimension. Mean intensity as a function of depth was then utilized to extract three different features, then fed to genetic algorithms to solve optimization problems to guide UR5e towards the optimal imaging plane.
Results and Conclusions: Genetic algorithms converged towards an average error < 1º after exploring only 18 positions, showing strong potential in automatic probe placement for LUS.
References
1. Fatima N, Mento F, Zanforlin A, Smargiassi A, Torri E, Perrone T, et al. Human-to-AI Interrater Agreement for Lung Ultrasound Scoring in COVID-19 Patients. J Ultrasound Med [Internet]. 2023 Apr 1;42(4):843–51. Available from: https://doi.org/10.1002/jum.16052
2. Fatima N, Khan U, Han X, Zannin E, Rigotti C, Cattaneo F, et al. Deep learning approaches for automated classification of neonatal lung ultrasound with assessment of human-to-AI interrater agreement. Comput Biol Med [Internet]. 2024;183:109315. Available from: https://doi.org/10.1016/j.compbiomed.2024.109315
3. Lerchbaumer MH, Lauryn JH, Bachmann U, Enghard P, Fischer T, Grune J, et al. Point-of-care lung ultrasound in COVID-19 patients: inter- and intra-observer agreement in a prospective observational study. Sci Rep [Internet]. 2021;11(1). Available from: https://doi.org/10.1038/s41598-021-90153-2
4. Mento F, Khan U, Faita F, Smargiassi A, Inchingolo R, Perrone T, et al. State of the Art in Lung Ultrasound, Shifting from Qualitative to Quantitative Analyses. Ultrasound Med Biol [Internet]. 2022;48(12):2398–416. Available from: https://doi.org/10.1016/j.ultrasmedbio.2022.07.007
5. Demi L, Wolfram F, Klersy C, De Silvestri A, Ferretti VV, Muller M, et al. New International Guidelines and Consensus on the Use of Lung Ultrasound. J Ultrasound Med [Internet]. 2023 Feb 1;42(2):309–44. Available from: https://doi.org/10.1002/jum.16088
6. Soldati G, Smargiassi A, Inchingolo R, Buonsenso D, Perrone T, Briganti DF, et al. Proposal for International Standardization of the Use of Lung Ultrasound for Patients With COVID-19. J Ultrasound Med [Internet]. 2020;39(7):1413–9. Available from: https://doi.org/10.1002/jum.15285
7. Tsumura R, Hardin JW, Bimbraw K, Grossestreuer A V, Odusanya OS, Zheng Y, et al. Tele-Operative Low-Cost Robotic Lung Ultrasound Scanning Platform for Triage of COVID-19 Patients. IEEE Robot Autom Lett [Internet]. 2021;6(3):4664–71. Available from: https://doi.org/10.1109/lra.2021.3068702
8. Tan J, Li B, Leng Y, Li Y, Peng J, Wu J, et al. Fully Automatic Dual-Probe Lung Ultrasound Scanning Robot for Screening Triage. IEEE Trans Ultrason Ferroelectr Freq Control [Internet]. 2023;70(9):975–88. Available from: https://doi.org/10.1109/tuffc.2022.3211532
9. Lei L, Hu Y, Jiang Z, Miao J, Luo X, Zhang Y, et al. Toward Lung Ultrasound Automation: Fully Autonomous Robotic Longitudinal and Transverse Scans Along Intercostal Spaces. IEEE Trans Med Robot Bionics [Internet]. 2025;7(2):768–81. Available from: https://doi.org/10.1109/TMRB.2025.3550663
10. Mento F, Perini M, Malacarne C, Demi L. Ultrasound multifrequency strategy to estimate the lung surface roughness, in silico and in vitro results. Ultrasonics [Internet]. 2023;135:107143. Available from: https://doi.org/10.1016/j.ultras.2023.107143
11. Blüthgen C, Sanabria S, Frauenfelder T, Klingmüller V, Rominger M. Economical Sponge Phantom for Teaching, Understanding, and Researching A- and B-Line Reverberation Artifacts in Lung Ultrasound. J Ultrasound Med [Internet]. 2017 Oct 1;36(10):2133–42. Available from: https://doi.org/10.1002/jum.14266
12. Mento F, Demi L. On the Influence of Imaging Parameters on Lung Ultrasound B-line Artifacts, in vitro study. J Acoust Soc Am [Internet]. 2020;148(2):975–83. Available from: https://doi.org/10.1121/10.0001797
13. Tortoli P, Bassi L, Boni E, Dallai A, Guidi F, Ricci S. ULA-OP: An Advanced Open Platform for Ultrasound Research. IEEE Trans Ultrason Ferroelectr Freq Control [Internet]. 2009 Oct 1;56:2207–16. Available from: https://doi.org/10.1109/TUFFC.2009.1303
14. Mento F, Soldati G, Prediletto R, Demi M, Demi L. Quantitative Lung Ultrasound Spectroscopy Applied to the Diagnosis of Pulmonary Fibrosis: The First Clinical Study. IEEE Trans Ultrason Ferroelectr Freq Control [Internet]. 2020;67(11):2265–73. Available from: https://doi.org/10.1109/TUFFC.2020.3012289
15. Xue W, Cao C, Liu J, Duan Y, Cao H, Wang J, et al. Modality alignment contrastive learning for severity assessment of COVID-19 from lung ultrasound and clinical information. Med Image Anal [Internet]. 2021;69. Available from: https://doi.org/10.1016/j.media.2021.101975
16. Custode LL, Mento F, Tursi F, Smargiassi A, Inchingolo R, Perrone T, et al. Multi-objective automatic analysis of lung ultrasound data from COVID-19 patients by means of deep learning and decision trees. Appl Soft Comput [Internet]. 2022;109926. Available from: https://doi.org/10.1016/j.asoc.2022.109926
17. Roshankhah R, Karbalaeisadegh Y, Greer H, Mento F, Soldati G, Smargiassi A, et al. Automated segmentation and scoring of lung ultrasound images of COVID-19 patients. J Acoust Soc Am [Internet]. 2020 Oct 1;148(4):2735. Available from: https://doi.org/10.1121/1.5147599
18. Chen J, He C, Yin J, Li J, Duan X, Cao Y, et al. Quantitative Analysis and Automated Lung Ultrasound Scoring for Evaluating COVID-19 Pneumonia with Neural Networks. IEEE Trans Ultrason Ferroelectr Freq Control [Internet]. 2021;68(7):2507–15. Available from: https://doi.org/10.1109/TUFFC.2021.3070696
19. Khan U, Mento F, Giacomaz LN, Trevisan R, Smargiassi A, Inchingolo R, et al. Deep Learning-based Classification of Reduced Lung Ultrasound Data from COVID-19 Patients. IEEE Trans Ultrason Ferroelectr Freq Control [Internet]. 2022;69(5):1661–9. Available from: https://doi.org/10.1109/TUFFC.2022.3161716
20. Khan U, Afrakhteh S, Mento F, Mert G, Smargiassi A, Inchingolo R, et al. Low-complexity lung ultrasound video scoring by means of intensity projection-based video compression. Comput Biol Med [Internet]. 2024;169:107885. Available from: https://doi.org/10.1016/j.compbiomed.2023.107885
21. Khokhlova TD, Thomas GP, Hall J, Steinbock K, Thiel J, Cunitz BW, et al. Development of an Automated Ultrasound Signal Indicator of Lung Interstitial Syndrome. J Ultrasound Med [Internet]. 2023 Dec 5; Available from: https://doi.org/10.1002/jum.16383
22. Tsai C-H, van der Burgt J, Vukovic D, Kaur N, Demi L, Canty D, et al. Automatic deep learning-based pleural effusion classification in lung ultrasound images for respiratory pathology diagnosis. Phys Medica [Internet]. 2021;83:38–45. Available from: https://doi.org/10.1016/j.ejmp.2021.02.023
23. Roy S, Menapace W, Oei S, Luijten B, Fini E, Saltori C, et al. Deep learning for classification and localization of COVID-19 markers in point-of-care lung ultrasound. IEEE Trans Med Imaging [Internet]. 2020;39(8):2676–87. Available from: https://doi.org/10.1109/TMI.2020.2994459
24. Mento F, Perpenti M, Barcellona G, Perrone T, Demi L. Lung Ultrasound Spectroscopy Applied to the Differential Diagnosis of Pulmonary Diseases: An In Vivo Multicenter Clinical Study. IEEE Trans Ultrason Ferroelectr Freq Control [Internet]. 2024;71(10):1217–32. Available from: https://doi.org/10.1109/TUFFC.2024.3454956
25. Jalilian H, Afrakhteh S, Mento F, Zannin E, Rigotti C, Cattaneo F, et al. Lung ultrasound video scoring using a novel motion-aware segmentation technique: Toward automated neonatal LUS scoring. Comput Biol Med [Internet]. 2025;198:111244. Available from: https://doi.org/10.1016/j.compbiomed.2025.111244
Issue
Section
License
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Transfer of Copyright and Permission to Reproduce Parts of Published Papers.
Authors retain the copyright for their published work. No formal permission will be required to reproduce parts (tables or illustrations) of published papers, provided the source is quoted appropriately and reproduction has no commercial intent. Reproductions with commercial intent will require written permission and payment of royalties.











